Research Areas
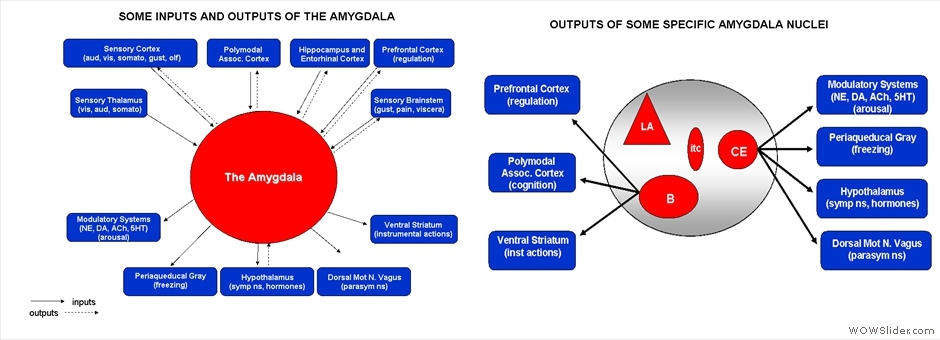
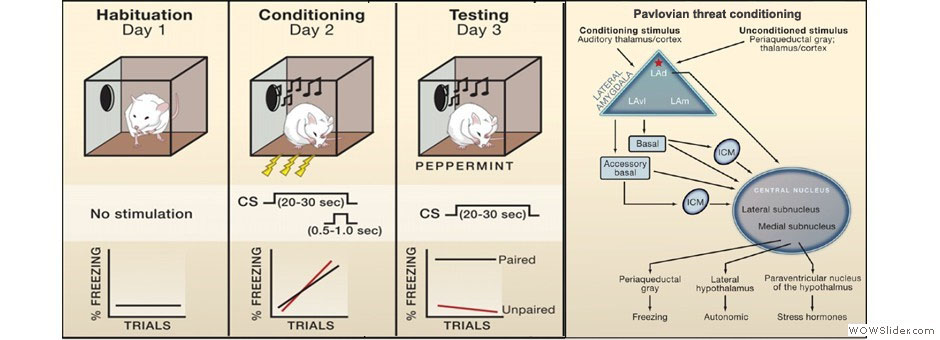
The LeDoux lab studies how the brain learns and stores information about danger using Pavlovian threat conditioning in rats. Specific circuits within the amygdala are essential for the formation of threat memories in both rats and humans. Our research involves a range of techniques, including pharmacological and viral-based manipulations, neuroanatomical tracing, and recordings of neural activity in awake and anesthetized animals.
 THE LEDOUX LAB
THE LEDOUX LAB 






BEHAVIOR
We use state-of-the-art neuroscientific techniques to examine how the brain processes threat stimuli and organizes defensive responses. We primarily study Pavlovian threat conditioning (commonly known as fear conditioning), and have recently begun to investigate how this kind of learning motivates Active Avoidance. By establishing solid behaviors with known neural correlates, we can disrupt these behaviors with various techniques including lesions, pharmacology, pharmacogenetics, and optogenetics, to examine the roles of specific structures and connections in the brain.
In Active Avoidance, an animal is given the ability to escape an aversive CS (signaled avoidance) or environment (Sidman avoidance) by shuttling from one side of a dual-compartment chamber to another or performing other target responses (escape from threat). We have also begun to study the integration of these processes in aversive Pavlovian-to-instrumental transfer. These learning paradigms allow us to disentangle the processes underlying how animals develop and display active behaviors to respond to threatening events.
ANATOMY
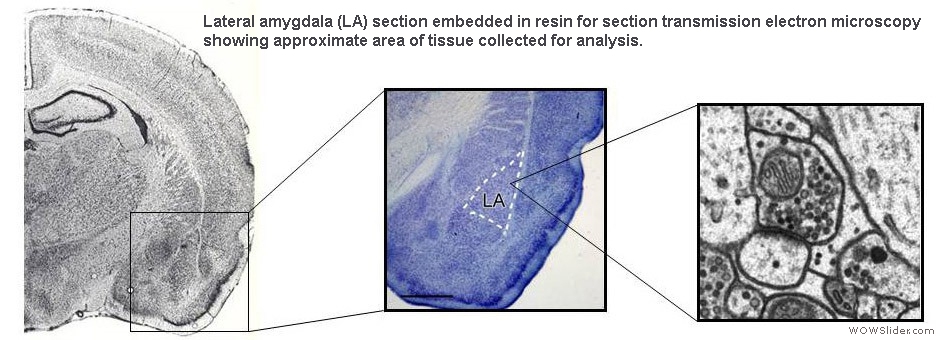
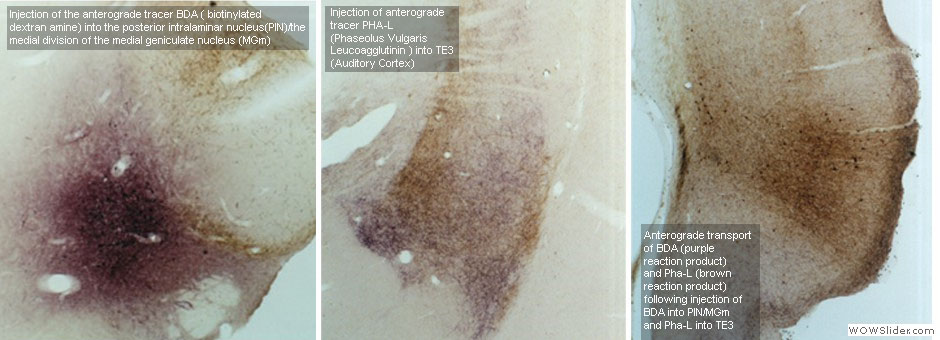
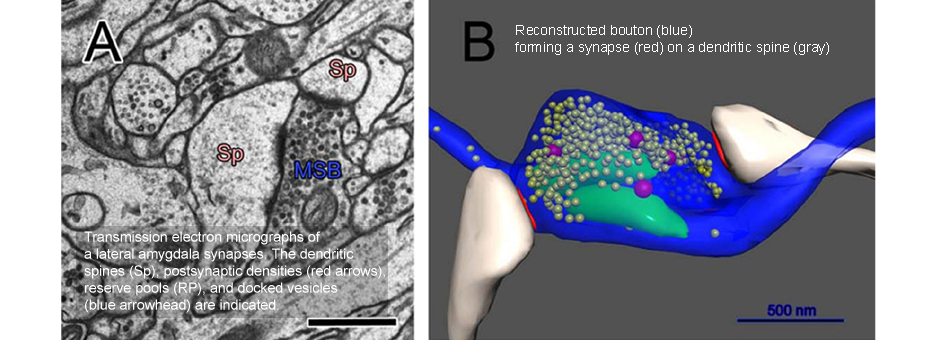
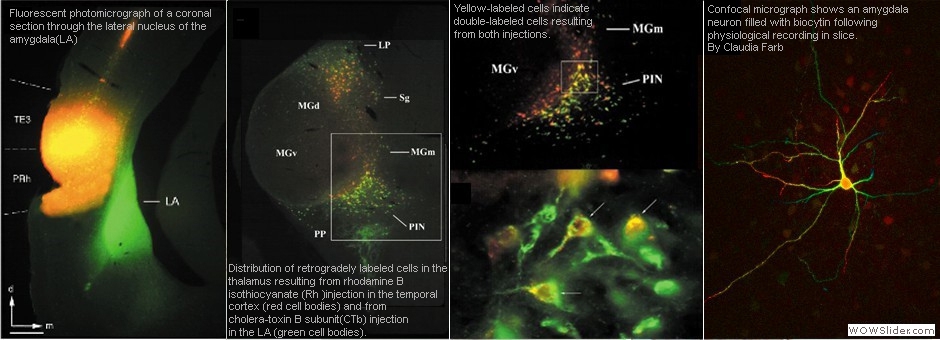
To understand how the amygdala processes aversive Pavlovian conditioning, our lab employs a variety of anatomical techniques to study the neuro-circuitry of the amygdala. We use tract tracing, in combination with electron microscopy, to examine the neural pathways to and from the amygdala, and to characterize the transmitters, receptors, proteins and types of synapses involved in aversive conditioning. Some of our studies combine behavioral manipulations and anatomical techniques to determine whether morphological changes take place in amygdala neurons following learning. For example, we used serial section electron microscopy and 3-D reconstruction, after animals underwent different forms of conditioning, to characterize the subcellular changes that occur in the dendritic spines of amygdala neurons. Our anatomical studies have contributed to understanding the circuitry of the amygdala and how it processes and integrates sensory information to execute specific behaviors.
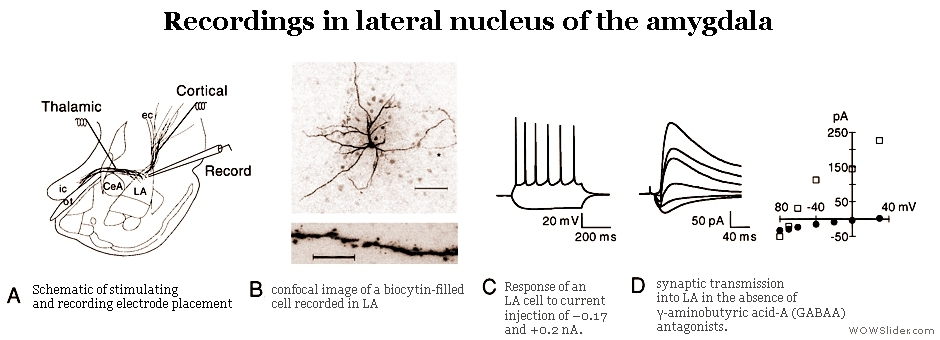
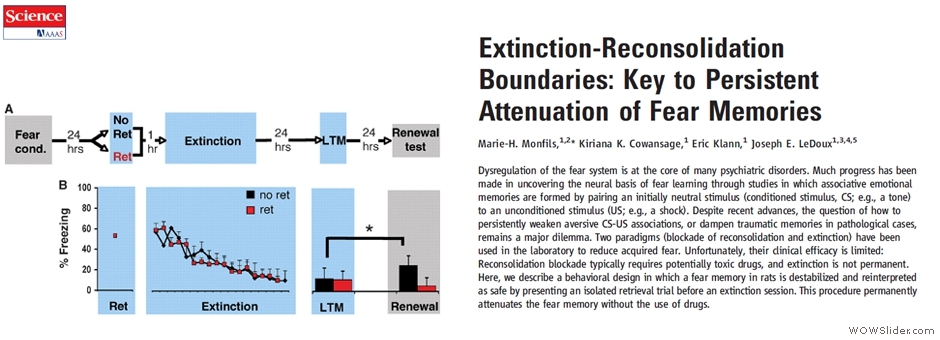
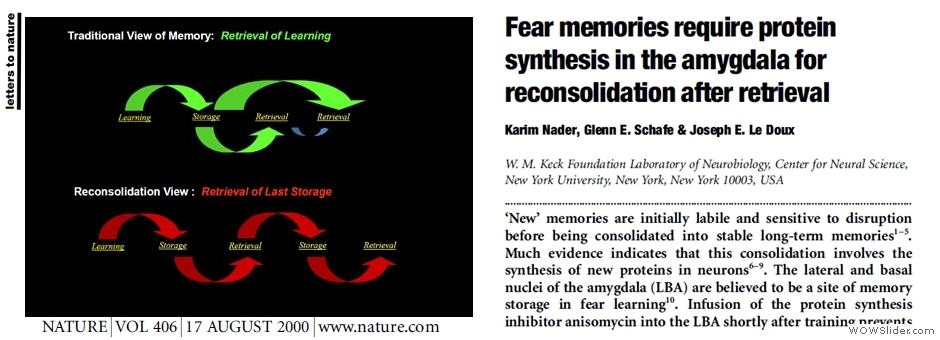
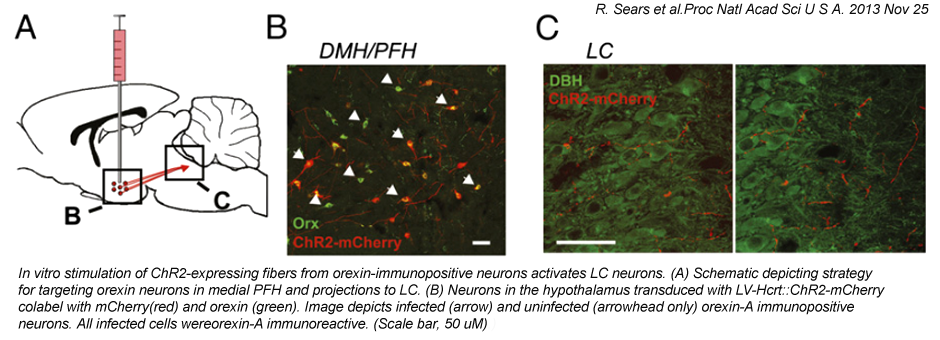
PHYSIOLOGY
Our physiological assays mainly involve the following:
Techniques used: pharmacological and viral manipulations, optogenetic manipulations
MOLECULAR MECHANISMS
How do molecular changes in the brain contribute to memory formation? Which proteins contribute to these changes? What are the molecular pathways involved in creating a memory? And when during behavior, will a molecular change affect learning or memory? To answer questions like these, we use a wide array of techniques with the aim of understanding the molecular mechanisms that underlie learning and memory of associative threat conditioning.
Using biochemical and immunohistochemical techniques, we assess molecular changes following behaviors of interest, sometimes in conjunction with pharmacological or genetic manipulations. We take a candidate approach to identify which molecular pathways are involved in threat memory formation. In collaboration with Dr. Stella Dracheva at Mt. Sinai School of Medicine, we also use cutting edge sequencing techniques to determine transcriptional changes after a memory is formed. Unlike the candidate approach, sequencing allows us to uncover novel players and pathways important for memory formation.
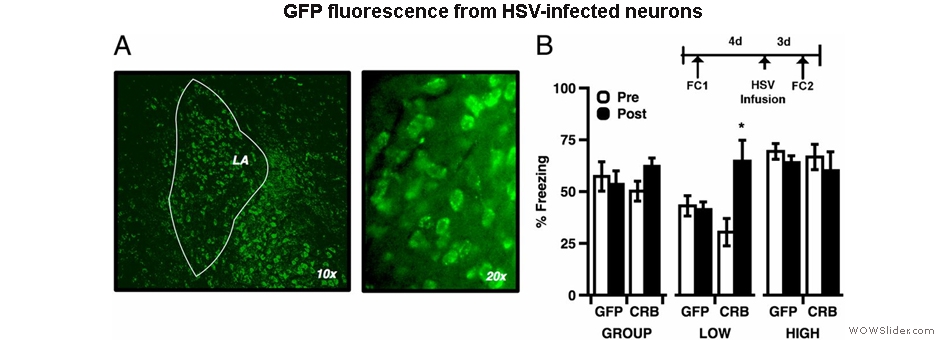
Using molecular biological techniques, we generate viral vectors to express exogenous genes in the brain or to remove endogenous gene expression. Specifically, using optogenetics, we express light-activated opsins to activate or inhibit neurons or neuronal projections in a temporally restricted manner. This allows us to determine when during the behavior specific neurons or neuronal projections are required for learning. We also use pharmacogenetics, or DREADD (designer receptors exclusively activated by designer drugs,) to activate or inhibit neurons over an extended timescale during behavior. Finally, we use RNAi (RNA interference) to knock down gene transcripts to determine the role of specific molecular players in memory formation.